
Chemistry, 05.05.2020 09:57 alyssagonzales2021
A balloon of air occupies 10.0L(V2)at25.0°C(T2)and1.00atm(P2) . What temperature (T1) was it initially, if it occupied 9.40 L (V1) and was in a freezer with a pressure of 0.939 atm (P1)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
A balloon of air occupies 10.0L(V2)at25.0°C(T2)and1.00atm(P2) . What temperature (T1) was it initial...
Questions

Computers and Technology, 25.11.2020 19:00




Geography, 25.11.2020 19:00

Mathematics, 25.11.2020 19:00

Mathematics, 25.11.2020 19:00


Mathematics, 25.11.2020 19:00


Mathematics, 25.11.2020 19:00




Arts, 25.11.2020 19:00

Biology, 25.11.2020 19:00





 = initial pressure of gas = 0.939 atm
= initial pressure of gas = 0.939 atm = final pressure of gas = 1.00 atm
= final pressure of gas = 1.00 atm = initial volume of gas = 9.40 L
= initial volume of gas = 9.40 L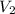 = final volume of gas = 10.0 L
= final volume of gas = 10.0 L = initial temperature of gas = ?
= initial temperature of gas = ? = final temperature of gas =
= final temperature of gas = 





