
Chemistry, 05.05.2020 10:36 witerose701
In the following reaction, how many moles of H2O are created when 7 moles of oxygen are consumed?
A.3 mol of CO2
B.5 mol of O2
C.72.08 grams of H2O
D.6 moles of H2O
F.1 mol of C3H8
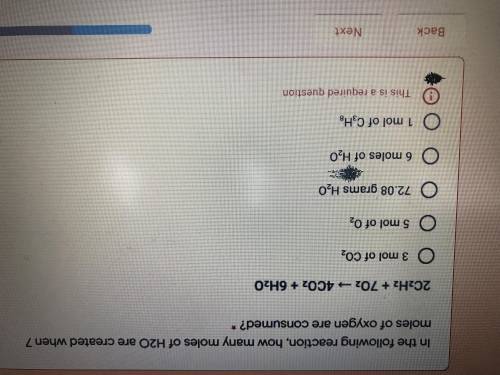

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
In the following reaction, how many moles of H2O are created when 7 moles of oxygen are consumed?
Questions



English, 19.12.2019 11:31

History, 19.12.2019 11:31

Mathematics, 19.12.2019 11:31

Social Studies, 19.12.2019 11:31

English, 19.12.2019 11:31


Geography, 19.12.2019 11:31



Mathematics, 19.12.2019 11:31

History, 19.12.2019 11:31

Biology, 19.12.2019 11:31

Mathematics, 19.12.2019 11:31

Mathematics, 19.12.2019 11:31

Mathematics, 19.12.2019 11:31



Chemistry, 19.12.2019 11:31

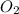 are used up to produce 6
are used up to produce 6


