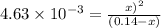Chemistry, 05.05.2020 08:58 Kategaldamez3
The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 527 °C
If 10 g of COCl2(g) is placed in a 1 L container, determine how much Cl2 is present at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 52...
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 52...
Questions

Mathematics, 22.09.2020 16:01



Mathematics, 22.09.2020 16:01



English, 22.09.2020 16:01


Arts, 22.09.2020 16:01

Computers and Technology, 22.09.2020 16:01



Mathematics, 22.09.2020 16:01

English, 22.09.2020 16:01

Biology, 22.09.2020 16:01

Health, 22.09.2020 16:01


Mathematics, 22.09.2020 16:01

Mathematics, 22.09.2020 16:01

Mathematics, 22.09.2020 16:01

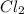 at equilibrium is 0.023 M
at equilibrium is 0.023 M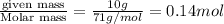
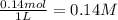
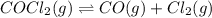
![K_c=\frac{[CO]\times [Cl_2]}{[COCl_2]}](/tpl/images/0635/7088/d6aec.png)