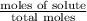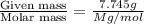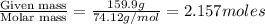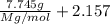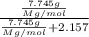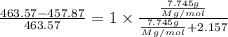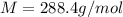The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. The vapor pressure of diethyl ether , CH3CH2OCH2CH3, is 463.57 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 7.745 grams of the compound were dissolved in 159.9 grams of diethyl ether, the vapor pressure of the solution was 457.87 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in...
Questions


History, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01




Mathematics, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01

Mathematics, 29.09.2020 07:01

English, 29.09.2020 07:01



English, 29.09.2020 07:01

Social Studies, 29.09.2020 07:01

Biology, 29.09.2020 07:01

Chemistry, 29.09.2020 07:01


Geography, 29.09.2020 07:01

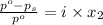
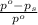 = relative lowering in vapor pressure
= relative lowering in vapor pressure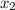 = mole fraction of solute =
= mole fraction of solute =