
Chemistry, 05.05.2020 08:19 iwannabewinston
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked to determine how many moles of water you can produce from 4.0 molmol of hydrogen and excess oxygen. (Excess oxygen means that so much oxygen is available it will not run out.) Which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2H2(g)+O2(g)→2H2O(l)2H2(g)+O2(g)→2H 2O(l) View Available Hint(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked...
Questions


Mathematics, 10.03.2021 01:00

English, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00


Geography, 10.03.2021 01:00

Biology, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

World Languages, 10.03.2021 01:00

Geography, 10.03.2021 01:00



Spanish, 10.03.2021 01:00

Physics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


History, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

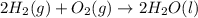
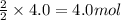 of water.
of water.

