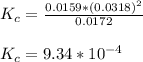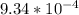Chemistry, 05.05.2020 07:20 stphdrn4347
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2 C l − ( a q ) PbClX2(s)↽−−⇀PbX2+(aq)+2ClX−(aq) Suppose you add 0.2393 g of P b C l 2 ( s ) PbClX2(s) to 50.0 mL of water. When the solution reaches equilibrium, you find that the concentration of P b 2 + ( a q ) PbX2+(aq) is 0.0159 M and the concentration of C l − ( a q ) ClX−(aq) is 0.0318 M. What is the value of the equilibrium constant, Kc, for the dissolution of P b C l 2 PbClX2?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What are the concentrations of hydroxide and hydronium ions in a solution with a ph of 10.2?
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2...
Questions




Computers and Technology, 16.04.2020 01:46

Biology, 16.04.2020 01:46


History, 16.04.2020 01:46









English, 16.04.2020 01:46

History, 16.04.2020 01:46

Social Studies, 16.04.2020 01:46


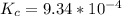
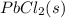 ⇌
⇌ 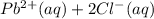
![[Pb^{2+}]](/tpl/images/0634/7278/0acfd.png) = 0.0159 M
= 0.0159 M
![[Cl^-]](/tpl/images/0634/7278/0726e.png) = 0.0318 M
= 0.0318 M
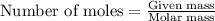
 Number of moles of PbCl₂
Number of moles of PbCl₂ 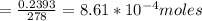
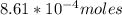
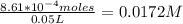
![K_c=\frac{[Pb^{2+}][Cl^-]}{[PbCl_2]} \\\\](/tpl/images/0634/7278/c4946.png)