
Chemistry, 05.05.2020 05:58 CoolRahim9090
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is an integer. If the hydrate is heated, the water can be drive off leaving pure CuSO4 behind. Suppose a sample of a certain hydrate is heated until all water is removed, and its found that the mass of the sample decreases by 31%. Which hydrate is it? THat is, WHAT IS X?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is a...
Questions




History, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

Chemistry, 30.06.2019 00:00

Advanced Placement (AP), 30.06.2019 00:00


Biology, 30.06.2019 00:00


Mathematics, 30.06.2019 00:00






English, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

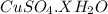
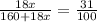
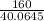 = 3.99 ≅ 4.
= 3.99 ≅ 4.

