Chemistry, 05.05.2020 06:08 ismailhajisaid29101
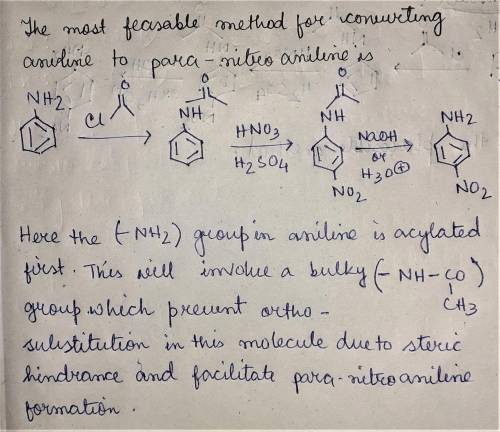
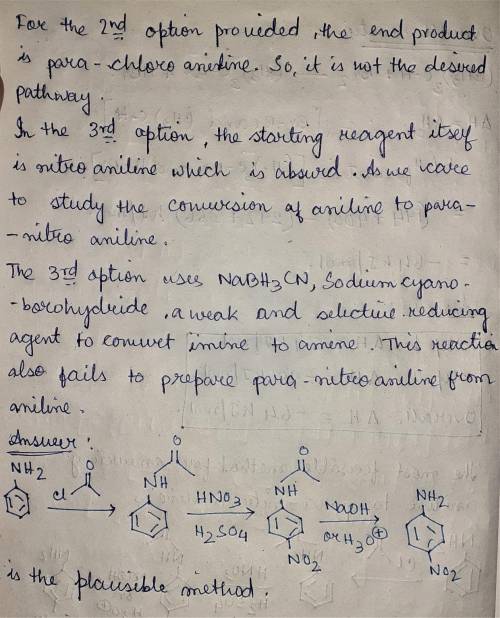
When aniline is treated with a mixture of nitric acid and sulfuric acid, the expected nitration product (para-nitroaniline) is obtained in poor yield. Instead, the major product from nitration is meta-nitroaniline. Apparently, the amino group is protonated under these acidic conditions, and the resulting ammonium group is a meta-director, rather than an ortho-para director. Propose a plausible method for converting aniline into para-nitroaniline.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
When aniline is treated with a mixture of nitric acid and sulfuric acid, the expected nitration prod...
Questions

Biology, 04.03.2021 17:00


Mathematics, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00








History, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00


Mathematics, 04.03.2021 17:00

Mathematics, 04.03.2021 17:00