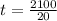Chemistry, 05.05.2020 06:35 genyjoannerubiera
Zinc is to be electroplated onto both sides of an iron sheet that is 20 cm2 as a galvanized sacrificial anode. It is desired to electroplate the zinc to a thickness of 0.025 mm. It is found that a current of 20 A produces a zinc coating of sufficient quality for galvanized iron. Determine the time required to produce the desired coating, assuming 100 % efficiency.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Zinc is to be electroplated onto both sides of an iron sheet that is 20 cm2 as a galvanized sacrific...
Questions




History, 29.01.2020 01:53

English, 29.01.2020 01:53

Arts, 29.01.2020 01:53




Mathematics, 29.01.2020 01:53

Mathematics, 29.01.2020 01:53

Social Studies, 29.01.2020 01:53




Mathematics, 29.01.2020 01:53

Mathematics, 29.01.2020 01:53

Biology, 29.01.2020 01:53


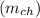 is given as :
is given as :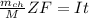
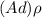 ; replacing that into above equation; we have:
; replacing that into above equation; we have: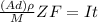 ---- equation (1)
---- equation (1)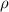 = density
= density ![[ \frac{20 cm^2 *0.0025 cm*7.13g/cm^3}{65.38g/mol}*2 \frac{moles\ of \ electrons}{mole \ of \ Zn} * 9.65*10^4 \frac{C}{mole \ of \ electrons } ] = (20 A) t](/tpl/images/0634/2762/37add.png)