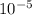Chemistry, 05.05.2020 05:14 QuestionAsker4356
The next 3 questions will walk you through using the Henderson-Hasselbalch equation for the following question. For each step provide a numerical answer. A solution is made by combining 300mL of 0.020M trimethyl amine, that is (CH3)3N and a 500mL of a 0.03M solution of it's conjugate acid, that is (CH3)3NH . The Kb for trimethyl amine is 6.3x10-5. The Henderson-Hasselbalch equation uses pKa not Kb, Calculate the pKa

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
The next 3 questions will walk you through using the Henderson-Hasselbalch equation for the followin...
Questions

Health, 02.02.2022 07:40



Mathematics, 02.02.2022 07:40

History, 02.02.2022 07:40


Chemistry, 02.02.2022 07:40

Biology, 02.02.2022 07:40





SAT, 02.02.2022 07:50





Computers and Technology, 02.02.2022 07:50


Social Studies, 02.02.2022 07:50