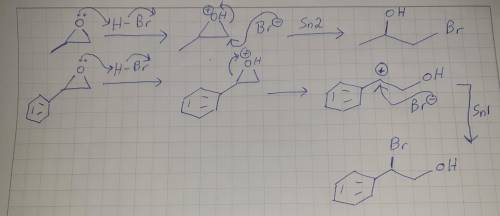
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. However, when phenyloxirane is treated with HBr, the bromide ion attacks the more substituted position. Explain the difference in regiochemistry in terms of a competition between steric effects and electronic effects. (Hint: It may help to draw out the structure of the phenyl group.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. Howev...
Questions

Mathematics, 22.09.2019 03:30


English, 22.09.2019 03:30


Mathematics, 22.09.2019 03:30

History, 22.09.2019 03:30







Biology, 22.09.2019 03:30


Mathematics, 22.09.2019 03:30

Mathematics, 22.09.2019 03:30

Business, 22.09.2019 03:30


Health, 22.09.2019 03:30

Mathematics, 22.09.2019 03:30