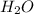Chemistry, 05.05.2020 04:06 asuhdude57
A website promoting the use of alternative energy vehicles and hybrid technologies claims that "A typical automobile in the USA uses about 40 gal of gasoline every month, producing about 750 lb of carbon dioxide." To determine the truth of this statement, calculate how many pounds of carbon dioxide are produced when 40.00 gal of gasoline are combusted. Assume that the primary ingredient in gasoline is octane, C8H18(l), which has a density of 0.703 g·mL−1.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A website promoting the use of alternative energy vehicles and hybrid technologies claims that "A ty...
Questions



Mathematics, 07.04.2020 22:10


Social Studies, 07.04.2020 22:10




Mathematics, 07.04.2020 22:10

Mathematics, 07.04.2020 22:10


Physics, 07.04.2020 22:10



Biology, 07.04.2020 22:10

Mathematics, 07.04.2020 22:10


Law, 07.04.2020 22:11

Mathematics, 07.04.2020 22:11

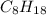 + 25
+ 25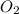 ------------> 16
------------> 16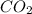 + 18
+ 18