Chemistry, 05.05.2020 04:10 carterkanye468
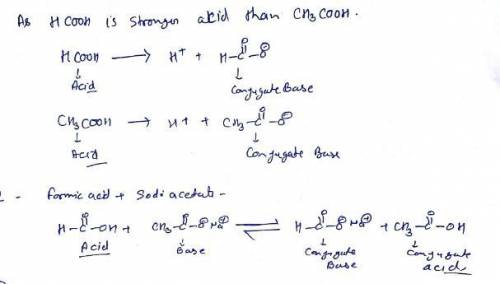
Given the following information: formic acid = HCOOH acetic acid = CH3COOH HCOOH is a stronger acid than CH3COOH (1) Write the net ionic equation for the reaction that occurs when equal volumes of 0.060 M aqueous formic acid and sodium acetate are mixed. It is not necessary to include states such as (aq) or (s). Use HCOO- as the formula for the formate ion.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Given the following information: formic acid = HCOOH acetic acid = CH3COOH HCOOH is a stronger acid...
Questions



Mathematics, 11.04.2020 03:47

Mathematics, 11.04.2020 03:47


Mathematics, 11.04.2020 03:47


Mathematics, 11.04.2020 03:47


Social Studies, 11.04.2020 03:47