Chemistry, 05.05.2020 04:13 Lizzy527663
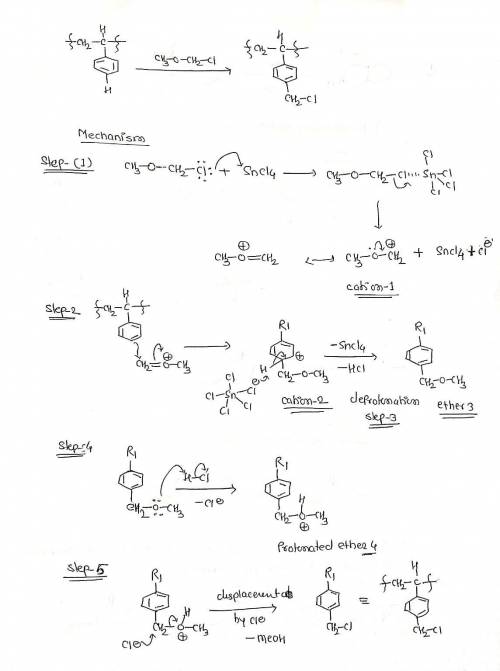
The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared by treatment of polystyrene with chloromethyl methyl ether and a Lewis acid catalyst. The reaction involves the following steps: Reaction of the ether with the Lewis acid to form cation 1; Electrophilic aromatic substitution to form resonance stabilized cation 2; Deprotonation yields aromatic ether 3; Protonation to form protonated ether 4; Displacement by chloride ion to form the final product. Write out the mechanism on a separate sheet of paper and then draw the structure of the resonance contributors of the resonance stabilized cation 2.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3


Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared...
Questions

Physics, 02.07.2020 08:01

English, 02.07.2020 08:01


Mathematics, 02.07.2020 08:01

Biology, 02.07.2020 08:01

Mathematics, 02.07.2020 08:01


Mathematics, 02.07.2020 08:01



Mathematics, 02.07.2020 08:01

Mathematics, 02.07.2020 08:01


Mathematics, 02.07.2020 08:01


Mathematics, 02.07.2020 08:01


Mathematics, 02.07.2020 08:01

English, 02.07.2020 08:01

Mathematics, 02.07.2020 08:01