
Chemistry, 05.05.2020 04:12 lilkobe6982
A compound contains 21.6% Na, 33.3% Cl, and 45.1% O. Write the empirical formula and name the compound that is formed

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
A compound contains 21.6% Na, 33.3% Cl, and 45.1% O. Write the empirical formula and name the compou...
Questions

Biology, 29.03.2021 19:30

Engineering, 29.03.2021 19:30


English, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30

Chemistry, 29.03.2021 19:30


Mathematics, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30

Spanish, 29.03.2021 19:30

History, 29.03.2021 19:30

Advanced Placement (AP), 29.03.2021 19:30

World Languages, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30

Social Studies, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30


English, 29.03.2021 19:30

Biology, 29.03.2021 19:30

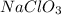 and the name of compound is sodium chlorate.
and the name of compound is sodium chlorate.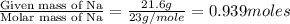

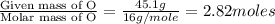

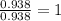
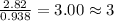
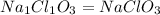 and the name of compound is sodium chlorate.
and the name of compound is sodium chlorate.

