Chemistry, 05.05.2020 04:13 Shayleechase
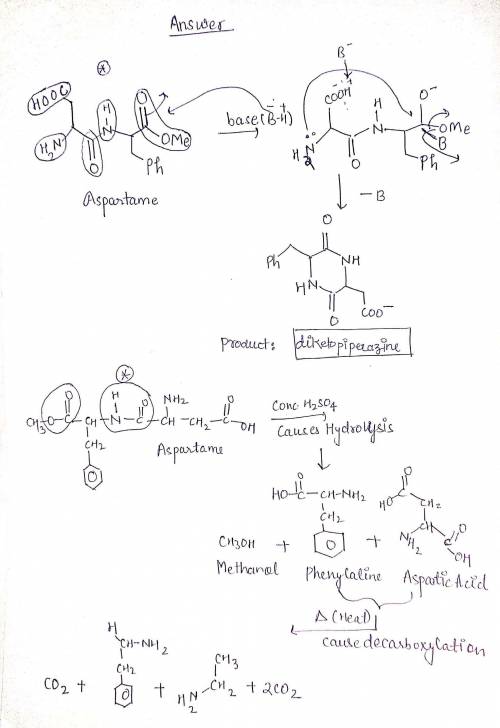
During this experiment, we exposed solutions of aspartame to base. What was the product of this reaction? Suppose you took your beverage containing aspartame and exposed it to concentrated H2SO4 and heat. What are products you might observe that you didn’t just mention? Which bond(s) are broken in the formation of these products? 3) This experiment relied on TLC and other qualitative tests. Why were IR or NMR spectroscopy not an option for analysis? 4) Which colors were observed after dipping your TLC plate in ninhydrin? Why were there multiple different colored products, and what functional groups did each colored product contain? What features of the starting material’s structure caused these colorimetric differenc

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
During this experiment, we exposed solutions of aspartame to base. What was the product of this reac...
Questions



Computers and Technology, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

English, 25.02.2021 22:40

Social Studies, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40







Law, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40



History, 25.02.2021 22:40