Which statement about Hfis true?
It is zero for any compound in its standard state.
It i...

Chemistry, 05.05.2020 03:09 wendyyy1214
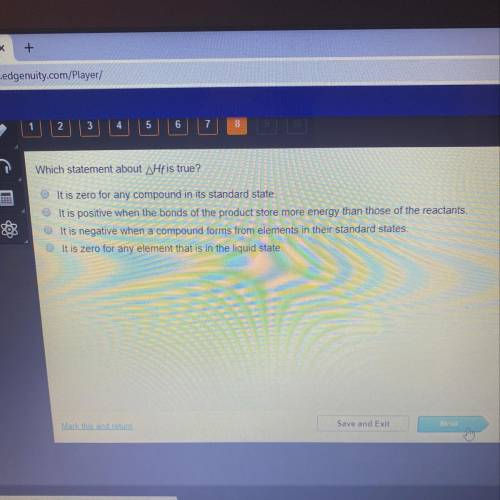
Which statement about Hfis true?
It is zero for any compound in its standard state.
It is positive when the bonds of the product store more energy than those of the reactants.
It is negative when a compound forms from elements in their standard states.
It is zero for any element that is in the liquid state.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Questions



English, 23.05.2021 14:40


Mathematics, 23.05.2021 14:40

Chemistry, 23.05.2021 14:40

Mathematics, 23.05.2021 14:40

History, 23.05.2021 14:40

English, 23.05.2021 14:40

English, 23.05.2021 14:40

Mathematics, 23.05.2021 14:40

Mathematics, 23.05.2021 14:40

Biology, 23.05.2021 14:40

History, 23.05.2021 14:50


Biology, 23.05.2021 14:50

English, 23.05.2021 14:50

Mathematics, 23.05.2021 14:50

English, 23.05.2021 14:50




