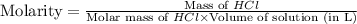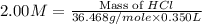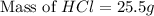Chemistry, 05.05.2020 03:17 aomoloju4202
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecular
weight of hydrochloric acid is 36.468
mol
What mass of hydrochloric acid (in grams) is needed for the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecul...
Questions

Mathematics, 19.04.2021 21:40

Mathematics, 19.04.2021 21:40







Mathematics, 19.04.2021 21:40




Mathematics, 19.04.2021 21:40


Mathematics, 19.04.2021 21:40

Mathematics, 19.04.2021 21:40



Mathematics, 19.04.2021 21:40

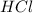 = 2.00 M
= 2.00 M