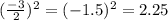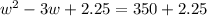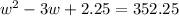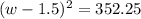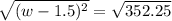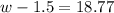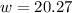Introduction: This activity introduces you to solutions and allows you to experience making different
concentrations of Kool-aid solution. There are many ways to calculate the concentration of a substance
including: molarity (M), parts per million (ppm), percent composition (% comp), and grams per liter (g/L). In
chemistry, concentration is usually measured by the number of moles of substance/ liter of substance, or
Molarity.
Materials:
• Kool-Aid Powder with SUGAR already included (or sugar if you don’t have kool-aid)
• Something to stir solutions
• 4 large cups
• measuring cup (ml preferred but cups will work)
Instructions: In this activity you will be making a series of kool-aid solutions. Make sure to record all data and
answer all the questions below.
Step 1: You will prepare a 237.5 ml kool-aid solution with a concentration of 1M. The molar mass of kool-aid
(or sugar since kool-aid is basically sugar C12H22O11.) is 342 g/mol. Show the calculations below which helped
you create your 1M solution. Note: 237.5 mL is approximately one cup.
Please show your work here.
Liters of water needed
Molarity of kool-aid needed
Grams of kool-aid needed
Now prepare the solution and make sure to label it (sticky note, pen on plastic cup, piece of paper under
cup, etc…)
To make this solution:
1. Measure approximately ½ dry cups of the Kool-aid mixture.
2. Pour the Kool-aid powder you measured into a large glass or cup.
3. Measure out the appropriate amount of water (1 cup) and mix into your glass/cup.
5. Stir to mix and label with the appropriate molarity.
6. Don’t taste test it yet, that will come later.
Do not discard the leftover 1M solution; we are not done with it yet!
Step 2: Using the 1M solution above you will create a second solution with a molarity of 0.5 M solution and
volume of 237.5 mL of kool-aid. Watch video link below for help.
Show your work here. You may need the following equation M1V1=M2V2 where M stands for molarity and V
standards for volume. :
Volume of 1M solution needed
Volume of water needed
Now prepare the solution and make sure to label it! Do not discard the leftover 1M and 0.5 M solutions;
we are not done with them yet!
Step 3: Using the 0.5 M solution above you will create 237.5 mL of a 0.25 M solution of kool-aid. You will need
the following equation M1V1=M2V2 where M stands for molarity and V standards for volume. Note: 237.5 ml is
about 1 cup. There is no video demonstration of this calculation, but follow the same dilution calculation
procedure as was demonstrated in step 2.
Show your work here:
Volume of 0.5 M solution needed:
Volume of water needed:
Now prepare the solution, make sure to label! Do not discard the leftover solutions; we are not done
with them yet!

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
Introduction: This activity introduces you to solutions and allows you to experience making differen...
Questions

Mathematics, 29.11.2019 18:31


Health, 29.11.2019 18:31



Biology, 29.11.2019 18:31


Mathematics, 29.11.2019 18:31


Mathematics, 29.11.2019 18:31


History, 29.11.2019 18:31


Health, 29.11.2019 18:31

Mathematics, 29.11.2019 18:31


Physics, 29.11.2019 18:31

Mathematics, 29.11.2019 18:31


Social Studies, 29.11.2019 18:31