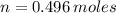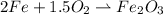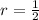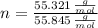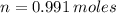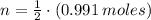Chemistry, 05.05.2020 00:28 bricksaspares
Iron and oxygen form rust. How many moles of rust should be produced if 1
55.321 grams of iron reacted? *

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3


Chemistry, 23.06.2019 15:30
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 16:30
Asample of calcium chloride (cacla) contains 100.2 grams of calcium (ca) and 177.3 grams of chlorine (co. what is the percent composition of chlorine 2.510% 36.10% 56.50% 63.89%
Answers: 2
You know the right answer?
Iron and oxygen form rust. How many moles of rust should be produced if 1
55.321 grams of iron...
55.321 grams of iron...
Questions

Mathematics, 05.03.2021 21:50

History, 05.03.2021 21:50


Mathematics, 05.03.2021 21:50


Mathematics, 05.03.2021 21:50





Mathematics, 05.03.2021 21:50


Chemistry, 05.03.2021 21:50

Chemistry, 05.03.2021 21:50

Mathematics, 05.03.2021 21:50

Mathematics, 05.03.2021 21:50


History, 05.03.2021 21:50


Mathematics, 05.03.2021 21:50