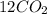When sugar is burned, water vapor and carbon dioxide are produced.
A sugar molecule has a specific number of carbon, hydrogen, and oxygen atoms. Use the balanced chemical equation to identify the number of carbon, hydrogen, and oxygen atoms in sugar.
Sugar + 1202 – 11H20 + 12C02
1.sugar has (11,12,22,24,34) carbon atoms?
2.sugar has (11,12,22,24,34) hydrogen atoms?
3.sugar has (11,12,22,24,34) oxygen atoms?
ANSWER QUESTIONS 1, 2, & 3
I WILL MAKE BRAINLIEST IF CORRECT(20 POINTS)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
When sugar is burned, water vapor and carbon dioxide are produced.
A sugar molecule has a speci...
A sugar molecule has a speci...
Questions


Mathematics, 02.09.2020 16:01





English, 02.09.2020 16:01




Mathematics, 02.09.2020 16:01

Social Studies, 02.09.2020 16:01






Business, 02.09.2020 16:01


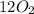 ⇒
⇒ 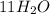 +
+