Chemistry, 03.05.2020 13:45 JamierW2005
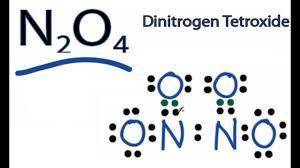
An oxide of nitrogen is 25.9% N by mass, has a molar mass of 108 g/mol, and contains no nitrogen-nitrogen or oxygen-oxygen bonds. Draw its Lewis structure (including all lone pair electrons) and name it. Include any nonzero formal charges in your structure.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
An oxide of nitrogen is 25.9% N by mass, has a molar mass of 108 g/mol, and contains no nitrogen-nit...
Questions

Mathematics, 10.05.2021 23:00

Social Studies, 10.05.2021 23:00

Mathematics, 10.05.2021 23:00




Mathematics, 10.05.2021 23:00



Mathematics, 10.05.2021 23:00


Computers and Technology, 10.05.2021 23:00

Mathematics, 10.05.2021 23:00

Mathematics, 10.05.2021 23:00


English, 10.05.2021 23:00



Health, 10.05.2021 23:00

History, 10.05.2021 23:00