12.Calculate the wavelength of an object moving with a velocity of 5 x105 m s-1
.(h = 6.6x10-3...

Chemistry, 03.05.2020 14:25 nabilop234
12.Calculate the wavelength of an object moving with a velocity of 5 x105 m s-1
.(h = 6.6x10-34 J s; mass of the object = 4 x10-22 kg)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
Questions


English, 07.02.2022 16:10

Biology, 07.02.2022 16:10

History, 07.02.2022 16:10



Mathematics, 07.02.2022 16:10

Mathematics, 07.02.2022 16:10




Chemistry, 07.02.2022 16:10


Mathematics, 07.02.2022 16:10


Law, 07.02.2022 16:10



Mathematics, 07.02.2022 16:10

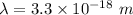
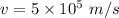
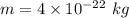
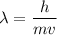
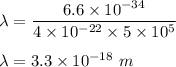
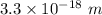 .
. 

