Chemistry, 05.05.2020 06:56 kcarstensen59070
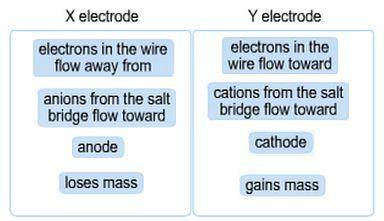
Attempt 4 A galvanic cell has an X electrode with X 2 plus ions in the left beaker and a Y electrode with Y 2 plus ions in the right beaker. A salt bridge connects the two halves. A galvanic (voltaic) cell has the generic metals X and Y as electrodes. X is more reactive than Y, that is, X more readily reacts to form a cation than Y does. Classify the descriptions by whether they apply to the X or Y electrode.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Attempt 4 A galvanic cell has an X electrode with X 2 plus ions in the left beaker and a Y electrode...
Questions




Spanish, 10.02.2021 19:30



Mathematics, 10.02.2021 19:30


Mathematics, 10.02.2021 19:30

Mathematics, 10.02.2021 19:30





History, 10.02.2021 19:30


Mathematics, 10.02.2021 19:30



Mathematics, 10.02.2021 19:30