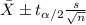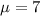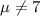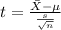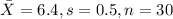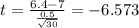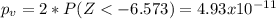Chemistry, 04.05.2020 22:37 lydia1melton
The Environmental Protection Agency has determined that safe drinking
water should have an average pH of 7 moles per liter. You are testing water from a new source, and take 30 vials of water. Water is unsafe if it deviates too far from 7 moles per liter in either direction. The mean pH level in your sample is 6.4 moles per liter, which is slightly acidic. The standard deviation of the sample is 0.5 moles per liter.
b) A 95% confidence interval for the true mean pH level of the water is (6.21, 6.59). Interpret this interval.
c) Explain why the interval in part (b) is consistent with the result of the test in part (a).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
The Environmental Protection Agency has determined that safe drinking
water should have an ave...
water should have an ave...
Questions

Mathematics, 19.03.2020 10:27

English, 19.03.2020 10:27

Chemistry, 19.03.2020 10:27

English, 19.03.2020 10:27


Mathematics, 19.03.2020 10:27

Mathematics, 19.03.2020 10:27



Physics, 19.03.2020 10:27





Mathematics, 19.03.2020 10:27

Chemistry, 19.03.2020 10:27

Mathematics, 19.03.2020 10:27

Mathematics, 19.03.2020 10:27

Mathematics, 19.03.2020 10:27