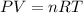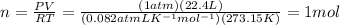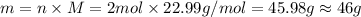Chemistry, 09.10.2019 09:30 nellyjsotelo
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4 l of h2 at stp? (the atomic mass of na is 22.99 a. 1.00 g. b. 2.00 g. c. 23.0 g. d. 46.0 g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

You know the right answer?
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4...
Questions




English, 01.12.2021 20:00

Mathematics, 01.12.2021 20:00



Social Studies, 01.12.2021 20:00



History, 01.12.2021 20:00

History, 01.12.2021 20:00

Geography, 01.12.2021 20:00

English, 01.12.2021 20:00



Geography, 01.12.2021 20:00

Computers and Technology, 01.12.2021 20:00

Computers and Technology, 01.12.2021 20:00

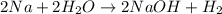
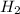 formed at STP,
formed at STP,