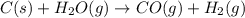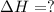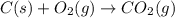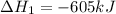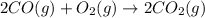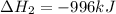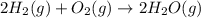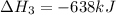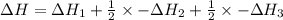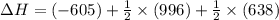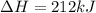Chemistry, 05.05.2020 14:47 clarajeansonels9987
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi
C(s) + H2O(g) - CO(g) + H2(9)
Given:
Reaction 1: C(s) + O2(g) – CO2(g) AHºrxn = -605 kJ
Reaction 2: 2 CO(g) + O2(g) – 2 CO2(g) AH°x = -966 kJ
Reaction 3: 2 H2(g) + O2(g) → 2 H2O(g) AHⓇx = -638 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Questions

Mathematics, 29.07.2019 06:10

Mathematics, 29.07.2019 06:10






Mathematics, 29.07.2019 06:10



English, 29.07.2019 06:10

Computers and Technology, 29.07.2019 06:10




Physics, 29.07.2019 06:10

History, 29.07.2019 06:10

Mathematics, 29.07.2019 06:10

Computers and Technology, 29.07.2019 06:10

Social Studies, 29.07.2019 06:10

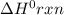 for the reaction is 212 kJ
for the reaction is 212 kJ