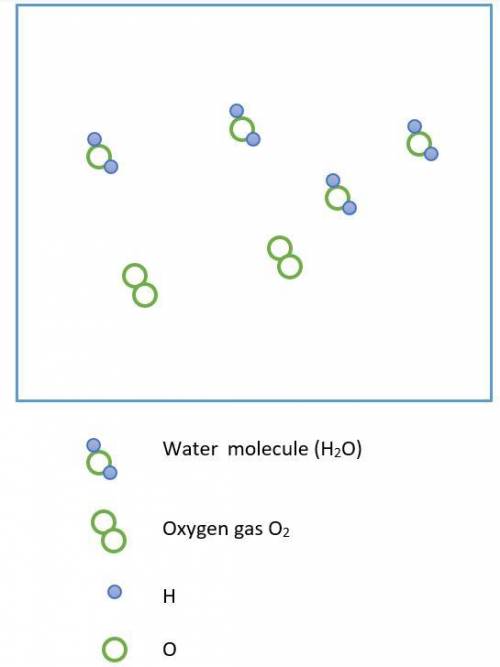
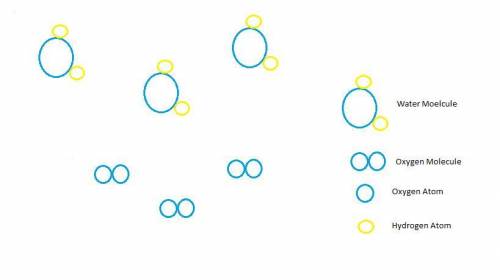
Chemistry, 05.05.2020 14:49 hosteenimport21
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamically favorable at room temperature.
(a) A particulate representation of the reactants is shown below in the box on the left. In the box below on the right, draw the particulate representation of all the molecules that would be produced from these four reactant molecules.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamica...
Questions

English, 28.03.2021 01:00




Mathematics, 28.03.2021 01:00

Social Studies, 28.03.2021 01:00


Physics, 28.03.2021 01:00


Computers and Technology, 28.03.2021 01:10



Mathematics, 28.03.2021 01:10


English, 28.03.2021 01:10


Mathematics, 28.03.2021 01:10



Spanish, 28.03.2021 01:10