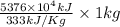On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass of ice you would need to add to bring the equilibrium temperature of the system to 300K. The pool contains 400,000 L (at a density of 1 kg/L) of water initially at 305K. Assume the ice is at 0°C (273K), the heat capacity of water is 4.2 J/(g*K), and the enthalpy of melting ice is 333 kJ/kg.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass...
Questions

Mathematics, 25.09.2020 01:01





Mathematics, 25.09.2020 01:01


Mathematics, 25.09.2020 01:01

History, 25.09.2020 01:01

Mathematics, 25.09.2020 01:01


Mathematics, 25.09.2020 01:01



Spanish, 25.09.2020 01:01

Business, 25.09.2020 01:01




Mathematics, 25.09.2020 01:01

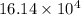 kg.
kg.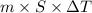
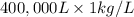
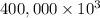 g (as 1 kg = 1000 g)
g (as 1 kg = 1000 g) = 305 K - 273 K
= 305 K - 273 K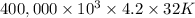
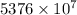 J
J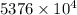 kJ
kJ