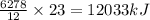Chemistry, 05.05.2020 00:44 Blakemiller2020
Using the following thermochemical equation, determine the amount of heat produced per kg of CO2 formed during the combustion of benzene (C6H6).
2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(g) ΔH°rxn = -6278 kJ

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
Using the following thermochemical equation, determine the amount of heat produced per kg of CO2 for...
Questions

Mathematics, 21.10.2020 01:01

English, 21.10.2020 01:01




Mathematics, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01

English, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01

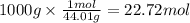
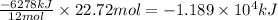
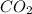 formed during the combustion of benzene
formed during the combustion of benzene 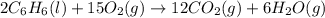
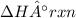 = -6278 kJ
= -6278 kJ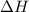 for the reaction comes out to be negative.
for the reaction comes out to be negative.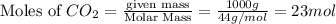 (1kg=1000g)
(1kg=1000g)