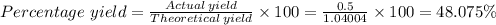100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following reaction: Al(NO3)3 + 3KOH → Al(OH)3 + 3KNO3
1) Identify the limiting reactant.
2) Identify the excess reactant.
3) Determine the theoretical yield of Al(OH)3
4) How much excess reactant is left over after the reaction is complete?
5) If 0.50g of Al(OH)3 determine the percent yield of Al(OH)3

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following...
the following...
Questions





Computers and Technology, 24.03.2021 01:20

Mathematics, 24.03.2021 01:20

English, 24.03.2021 01:20

Social Studies, 24.03.2021 01:20


Mathematics, 24.03.2021 01:20

Geography, 24.03.2021 01:20

Mathematics, 24.03.2021 01:20

Mathematics, 24.03.2021 01:20

Social Studies, 24.03.2021 01:20

History, 24.03.2021 01:20



Mathematics, 24.03.2021 01:20


Medicine, 24.03.2021 01:20