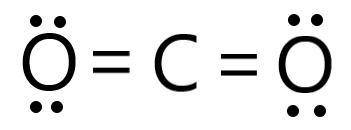Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Determine the electron geometry (eg) and molecular geometry (mg) of CO2.
eg=trigonal planar, mg...
eg=trigonal planar, mg...
Questions





Mathematics, 06.12.2019 01:31


Geography, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31

Social Studies, 06.12.2019 01:31


Mathematics, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31


Computers and Technology, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31