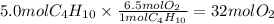Chemistry, 05.05.2020 09:45 michaelmorrison37
Butane, C4H10, is the gas burned in disposable lighters. How many moles of oxygen are needed to burn 5.0 mol of butane in a lighter to produce carbon dioxide and water?
Unbalanced equation:
C4H10 + O2 -> CO2 + H2O
28 mol
32 mol
13 mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1



Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
You know the right answer?
Butane, C4H10, is the gas burned in disposable lighters. How many moles of oxygen are needed to burn...
Questions


Mathematics, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30



Mathematics, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30




Mathematics, 30.04.2021 01:30

Computers and Technology, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30


Mathematics, 30.04.2021 01:30

Mathematics, 30.04.2021 01:30

Geography, 30.04.2021 01:30