
Chemistry, 05.05.2020 16:44 willwhitlock803
A critical reaction in the production of energy in biological systems is the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) as described by ATP(aq) + H2O(l) ADP(aq) + HPO4-2(aq) for which ΔG°rxn = –30.5 kJ/mol at 37.0°C. Calculate the value of ΔGrxn in a biological cell in which [ATP] = 1.2 x10-2 M, [ADP] = 8.4 x10-3 M, and [HPO4-2] = 2.1x10-3 M.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

You know the right answer?
A critical reaction in the production of energy in biological systems is the hydrolysis of adenosine...
Questions

Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10




Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10


Physics, 12.04.2021 21:10








English, 12.04.2021 21:10

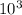 J/mol
J/mol ![\frac{[HPO4-2] x [ADP]}{ATP}](/tpl/images/0640/3718/8cebd.png)
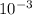 M
M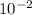 M
M![\frac{[2.1x10^{-3}] x [8.4x10^{-3}] }{[1.2 x 10^{-2}] }](/tpl/images/0640/3718/55eb3.png)


