Chemistry, 05.05.2020 17:37 canyonmorlan
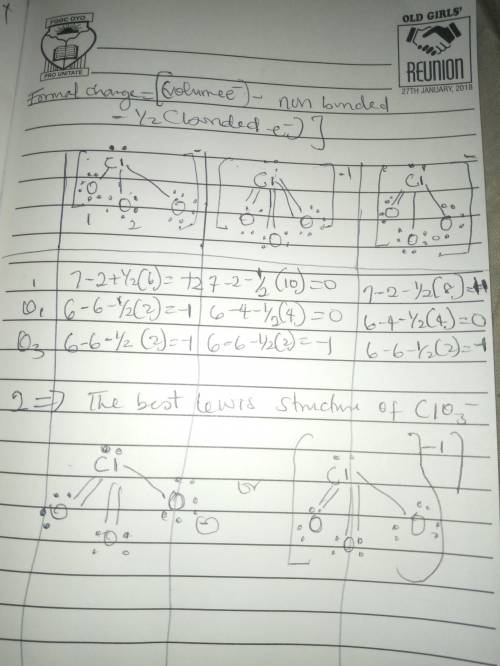
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the thiocyanate ion , SCN- . The concepts of formal charge and electronegativity can help us choose the structure that is the best representation.1. Assign formal charges to the elements in each of the structures below. ABCFormal ChargeSCN-12010-100-12. The best Lewis structure for SCN- is .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding e...
Questions


Physics, 22.10.2020 18:01


Health, 22.10.2020 18:01


History, 22.10.2020 18:01

History, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

English, 22.10.2020 18:01



Social Studies, 22.10.2020 18:01

Health, 22.10.2020 18:01


Chemistry, 22.10.2020 18:01

Biology, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

Biology, 22.10.2020 18:01