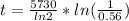Chemistry, 05.05.2020 17:41 tyresharichardson29
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was being dug for a new house, is 56.0% of the same ratio in a growing tree today. Assuming the ratio of carbon-14 to carbon-12 in the atmosphere has been constant, calculate the age of the arrow. The half-life of carbon-14 is 5730 years. The arrow is years old Numeric

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was...
Questions


Mathematics, 15.12.2021 15:50

English, 15.12.2021 15:50




Mathematics, 15.12.2021 15:50







Mathematics, 15.12.2021 15:50

English, 15.12.2021 15:50


English, 15.12.2021 15:50


Mathematics, 15.12.2021 16:00

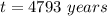 old
old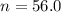 %
%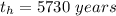
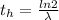
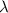 is the decay constant
is the decay constant 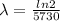
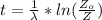
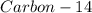 is
is 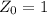
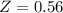 %
%