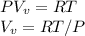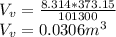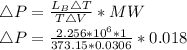Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling point of water by 1° C? You may assume that these changes are both small, so you only need to compute first derivatives. Remember that, under most conditions, the volume (per molecule) of liquid water is small compared to that of water vapor. Remember: Atmospheric pressure = 101300 Pa Boiling point at atmospheric pressure = 373.15 K Latent heat (LB) of boiling water = 2.256 × 106 J/kg Molecular weight (mw) of water = 0.018 kg/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling...
Questions

English, 23.03.2021 17:10

History, 23.03.2021 17:10


History, 23.03.2021 17:10


Mathematics, 23.03.2021 17:10

Mathematics, 23.03.2021 17:10


Mathematics, 23.03.2021 17:10

Mathematics, 23.03.2021 17:10



Mathematics, 23.03.2021 17:10


Health, 23.03.2021 17:10

Physics, 23.03.2021 17:10

History, 23.03.2021 17:10


Mathematics, 23.03.2021 17:10

English, 23.03.2021 17:10

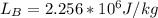
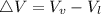
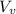 ) is far greater than the volume of liquid (
) is far greater than the volume of liquid (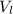 )
)