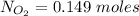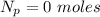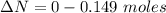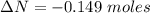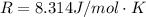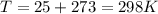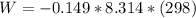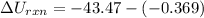The oxidation of copper(I) oxide, Cu2O(s) , to copper(II) oxide, CuO(s) , is an exothermic process. 2Cu2O(s)+O2(g)⟶4CuO(s) The change in enthalpy upon reaction of 42.60 g Cu2O(s) is −43.47 kJ . Calculate the work, , and energy change, Δrxn , when 42.60 g Cu2O(s) is oxidized at a constant pressure of 1.00 bar and a constant temperature of 25∘ C . Note that ΔErxn is sometimes used as the symbol for energy change instead of Δrxn .

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
The oxidation of copper(I) oxide, Cu2O(s) , to copper(II) oxide, CuO(s) , is an exothermic process....
Questions



English, 09.03.2021 16:00



Mathematics, 09.03.2021 16:00



Biology, 09.03.2021 16:00

Business, 09.03.2021 16:00

History, 09.03.2021 16:00

Mathematics, 09.03.2021 16:00



Biology, 09.03.2021 16:10


Advanced Placement (AP), 09.03.2021 16:10



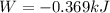
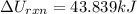

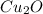 is
is 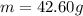
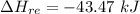 this also the change in energy in terms of heat
this also the change in energy in terms of heat 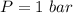
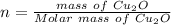
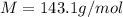
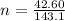
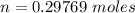
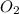 to give Four moles of
to give Four moles of 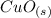
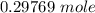 of
of 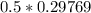 moles of
moles of 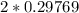 moles of
moles of 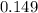 moles of
moles of 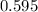 moles of
moles of