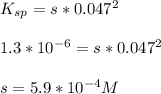Chemistry, 05.05.2020 18:43 alishabhappy1
Compute the moles/liter solubility of calcium hydroxide in 0.047 M sodium hydroxide. Ksp= 1.3 x 10^-6

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Compute the moles/liter solubility of calcium hydroxide in 0.047 M sodium hydroxide. Ksp= 1.3 x 10^-...
Questions

Social Studies, 13.04.2021 06:30






Chemistry, 13.04.2021 06:30

Mathematics, 13.04.2021 06:30

Social Studies, 13.04.2021 06:30

Chemistry, 13.04.2021 06:30

Mathematics, 13.04.2021 06:30

Mathematics, 13.04.2021 06:30

Chemistry, 13.04.2021 06:30



History, 13.04.2021 06:30

Geography, 13.04.2021 06:30




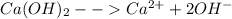
![K_{sp}= [Ca^{2+}][OH^{-}]^{2}](/tpl/images/0641/4157/18564.png)
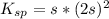
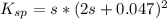 ,
,