
Calculate the potential of the electrochemical cell and determine if it is spontaneous as written at 25 ∘C . Cu(s) || Cu2+(0.14 M) ‖‖ Fe2+(0.0040 M) || Fe(s) E∘Cu2+/Cu=0.339 VE∘Fe2+/Fe=−0.440 V Ecell= V Is the electrochemical cell spontaneous or not spontaneous as written at 25 ∘C ? not spontaneous spontaneous Calculate the potential of the electrochemical cell and determine if it is spontaneous as written at 25 ∘C . Pt(s) || Sn2+(0.0067 M),Sn4+(0.11 M) ‖‖ Fe3+(0.15 M),Fe2+(0.0019 M) || Pt(s) E∘Sn4+/Sn2+=0.154 VE∘Fe3+/Fe2+=0.771 V Ecell= V Is the electrochemical cell spontaneous or not spontaneous as written at 25 ∘C ? spontaneous not spontaneous

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
Calculate the potential of the electrochemical cell and determine if it is spontaneous as written at...
Questions

Mathematics, 23.01.2020 13:31






English, 23.01.2020 13:31



Advanced Placement (AP), 23.01.2020 13:31



Mathematics, 23.01.2020 13:31

History, 23.01.2020 13:31

History, 23.01.2020 13:31




History, 23.01.2020 13:31

Mathematics, 23.01.2020 13:31

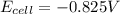
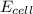 is negative
is negative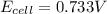
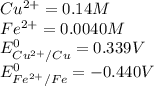
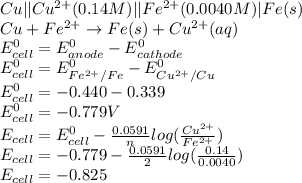
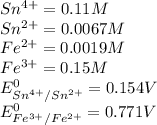
![Pt || Sn^{2+} (0.0067 M) || Sn^{4+} (0.11 M) | Fe^{3+}(0.15 M)\\Sn^{2+} + 2Fe^{3+} \rightarrow Sn^{4+} + 2Fe^{2+} (aq)\\ E_{cell} ^{0} = E_{anode} ^{0} - E_{cathode} ^{0}\\E_{cell} ^{0} = E_{Fe^{3+}/Fe^{2+} } ^{0} - E_{Sn^{4+}/Sn{2+}} ^{0}\\E_{cell} ^{0} = 0.771 - 0.154\\E_{cell} ^{0} = 0.617 V\\E_{cell} = E_{cell} ^{0} - \frac{0.0591}{n} log(\frac{[Fe^{2+}]^2 [Sn^{4+}]}{[Fe^{3+}]^2 [Sn^{2+}] } )\\E_{cell} = 0.617 - \frac{0.0591}{2} log(\frac{0.0019^2 * 0.11}{0.15^2 * 0.154 } )\\E_{cell} = 0.733 V](/tpl/images/0642/3814/7df54.png)


