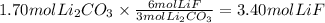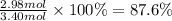The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate and lithium fluoride:
2AlF3 + 3Li2CO3 → Al2(CO3)3 + 6LiF.
You have an excess of aluminum trifluoride and 1.70 moles of lithium carbonate, which produces 2.98 moles of lithium fluoride. What is the percent yield of the reaction? Use the periodic table and this polyatomic ion resource.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate...
Questions


Mathematics, 31.01.2020 07:58

Social Studies, 31.01.2020 07:58

Computers and Technology, 31.01.2020 07:58

Mathematics, 31.01.2020 07:58

English, 31.01.2020 07:58

Chemistry, 31.01.2020 07:58

History, 31.01.2020 07:58

Mathematics, 31.01.2020 07:58


Mathematics, 31.01.2020 07:58


Mathematics, 31.01.2020 07:58


Mathematics, 31.01.2020 07:58

Mathematics, 31.01.2020 07:58


History, 31.01.2020 07:58


History, 31.01.2020 07:58