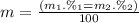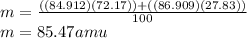Chemistry, 27.08.2019 07:00 Jerrikasmith28
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.
note that 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17% while 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.<...
Questions

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00



Mathematics, 22.03.2021 01:00

Spanish, 22.03.2021 01:00




Mathematics, 22.03.2021 01:00


Chemistry, 22.03.2021 01:00



English, 22.03.2021 01:00





Computers and Technology, 22.03.2021 01:00