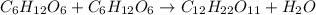Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equat...

Chemistry, 12.01.2020 01:31 mikayleighb2019
Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equation is not balanced; it is missing a molecule of water. write it in on the correct side of the equation.
b. what kind of reaction is this?
c. is c6h12o6 (glucose) a monomer, or a polymer?
d. to summarize, when two monomers are joined, a molecule of is always removed.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
Questions

Mathematics, 21.03.2020 21:39

Mathematics, 21.03.2020 21:39

Mathematics, 21.03.2020 21:39


Law, 21.03.2020 21:39




Computers and Technology, 21.03.2020 21:40

Mathematics, 21.03.2020 21:40





History, 21.03.2020 21:41



Mathematics, 21.03.2020 21:42

Mathematics, 21.03.2020 21:43

Social Studies, 21.03.2020 21:43