Given the equation representing a system at equilibrium in a sealed, rigid container:
2HI(g) &...

Given the equation representing a system at equilibrium in a sealed, rigid container:
2HI(g) <->H2(g) + I2(g) + energy
Increasing the temperature of the system causes the concentration of
1)HI to increase
2)H2 to increase
3) HI to Remain constant
4) HI to Remain constant

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
Questions



Business, 16.02.2021 07:20

History, 16.02.2021 07:20


Mathematics, 16.02.2021 07:20


Biology, 16.02.2021 07:20

Mathematics, 16.02.2021 07:20

English, 16.02.2021 07:20

Social Studies, 16.02.2021 07:20




Social Studies, 16.02.2021 07:20

Mathematics, 16.02.2021 07:20


Mathematics, 16.02.2021 07:20

Mathematics, 16.02.2021 07:20

English, 16.02.2021 07:20

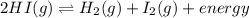
 will increase.
will increase.

