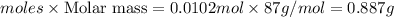Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq)HCl(aq), as described by the chemical equation MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g) MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g) How much MnO2(s)MnO2(s) should be added to excess HCl(aq)HCl(aq) to obtain 235 mL Cl2(g)235 mL Cl2(g) at 25 °C and 805 Torr805 Torr?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions


Mathematics, 09.11.2020 20:30

Biology, 09.11.2020 20:30


Computers and Technology, 09.11.2020 20:30



Mathematics, 09.11.2020 20:30


English, 09.11.2020 20:30


Social Studies, 09.11.2020 20:30

Mathematics, 09.11.2020 20:30







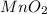 should be added to excess HCl(aq).
should be added to excess HCl(aq).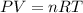
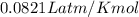
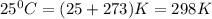
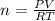
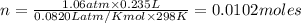
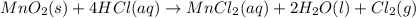
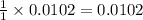 moles of
moles of