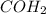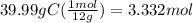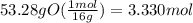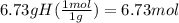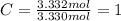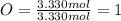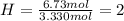Chemistry, 06.05.2020 07:45 jesh0975556
Determine the empirical formula of a compound that is 39.99% carbon, 53.28% Oxygen and 6.73% Hydrogen

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Determine the empirical formula of a compound that is 39.99% carbon, 53.28% Oxygen and 6.73% Hydroge...
Questions



Chemistry, 11.06.2021 04:30


Computers and Technology, 11.06.2021 04:30



Mathematics, 11.06.2021 04:30


Mathematics, 11.06.2021 04:30




Mathematics, 11.06.2021 04:30

History, 11.06.2021 04:30

Mathematics, 11.06.2021 04:30



Mathematics, 11.06.2021 04:30

Mathematics, 11.06.2021 04:30