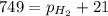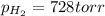Chemistry, 06.05.2020 17:08 22katelynfrankouqqrb
As the magnesium reacts, the hydrogen gas produced is collected by water displacement at 23.0oC. The pressure of the gas in the collection tube is measured to be 749 torr. Given that the equilibrium vapor pressure of water is 21 torr at 23.0oC, calculate the pressure that the H2(g) produced in the reaction would have if it were dry.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
As the magnesium reacts, the hydrogen gas produced is collected by water displacement at 23.0oC. The...
Questions

Mathematics, 05.10.2020 15:01

History, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01



Mathematics, 05.10.2020 15:01

Geography, 05.10.2020 15:01

Geography, 05.10.2020 15:01


Computers and Technology, 05.10.2020 15:01

Arts, 05.10.2020 15:01

History, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Social Studies, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Geography, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

 produced in the reaction would have if it were dry will be 728 torr
produced in the reaction would have if it were dry will be 728 torr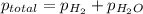
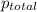 =total pressure of gas = 749 torr
=total pressure of gas = 749 torr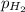 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?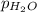 = partial pressure of water = 21 torr
= partial pressure of water = 21 torr