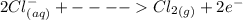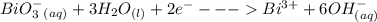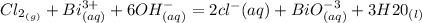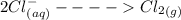Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq)...

Chemistry, 06.05.2020 20:11 owlette2001
Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq) = 2cl-(aq) + BiO-3 (aq) + 3H20 (l)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
Questions

English, 18.10.2019 07:10



Biology, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

History, 18.10.2019 07:10

History, 18.10.2019 07:10


Biology, 18.10.2019 07:10

English, 18.10.2019 07:10




Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

English, 18.10.2019 07:10