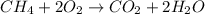Chemistry, 06.05.2020 23:07 Trucofer2106
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylene is reacted with O2, 18.5 g of water is formed.
2 C2H2 (g) + 3 O2 (g) → 2 CO2 (g) + 2 H2O (l)
What type of reaction is this?
What is the percent yield of this reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3


Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

You know the right answer?
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylen...
Questions


History, 13.07.2019 04:00

Mathematics, 13.07.2019 04:00


Biology, 13.07.2019 04:00


Mathematics, 13.07.2019 04:00

Health, 13.07.2019 04:00

Health, 13.07.2019 04:00

Mathematics, 13.07.2019 04:00



Mathematics, 13.07.2019 04:00

English, 13.07.2019 04:00

History, 13.07.2019 04:00

History, 13.07.2019 04:00


Mathematics, 13.07.2019 04:00


History, 13.07.2019 04:00