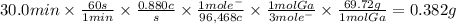Gallium is produced by the electrolysis of a solution made by dissolving gallium oxide in concentrated NaOH ( aq ) . NaOH(aq). Calculate the amount of Ga ( s ) Ga(s) that can be deposited from a Ga ( III ) Ga(III) solution using a current of 0.880 A 0.880 A that flows for 30.0 min .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1
You know the right answer?
Gallium is produced by the electrolysis of a solution made by dissolving gallium oxide in concentrat...
Questions

Social Studies, 17.07.2019 14:30


Social Studies, 17.07.2019 14:30


Biology, 17.07.2019 14:30

Biology, 17.07.2019 14:30

Biology, 17.07.2019 14:30

History, 17.07.2019 14:30


Mathematics, 17.07.2019 14:30



Biology, 17.07.2019 14:30

Mathematics, 17.07.2019 14:30

Chemistry, 17.07.2019 14:30



History, 17.07.2019 14:30

Chemistry, 17.07.2019 14:30

Social Studies, 17.07.2019 14:30